Past Issues
Green Synthesis of Silver Nanoparticles Using Phytochemicals and Encapsulation Using Synthetic Polymer (PVA)
Inchara S, Aaditya Vaidya, Mayur Patil, Kavitha SH, Dinesh MS*
Department of Biotechnology, PES University, India
*Corresponding author: Dr. MS Dinesh, Associate Professor of Biotechnology Department, PES University, Banglore, India, E-mail: [email protected]
Received Date: November 08, 2024
Publication Date: December 16, 2024
Citation: Inchara S, et al. (2024). Green Synthesis of Silver Nanoparticles Using Phytochemicals and Encapsulation Using Synthetic Polymer (PVA). Nanoparticle. 5(1):16.
Copyright: Inchara S, et al. © (2024).
ABSTRACT
Nanomaterials are one of the most researched topics due to the fact that the nano-range materials (1 - 100 nm) have stark differences compared to properties of the same materials in bulk dimensions. Silver nanoparticles (Ag-Np) have been researched because of their strong action against a range of microorganisms. Physical and chemical methods like chemical reduction and microwave irradiation are used to synthesize these nanoparticles. These methods, however, are dangerous and expensive. Green synthesis provides a safer, environmental-friendly, and cost effective way for synthesizing Ag-NPs. Green synthesized Ag-NPs have a multitude of applications such as water filtration, sanitisation and so on. They are also effective antibacterial agents against Gram positive and Gram negative bacteria. Plants and their various parts have been used in the synthesis of Ag-NPs due to their ability to act as reducing and stabilizing agents. PVA encapsulated Ag-NPs are said to be stable for several months and can be stored at room temperature. Nanoparticles need to be stabilized for any practical application and this can be done with the help of polymers such as polyvinyl alcohol (PVA). The characterisation of the nanoparticles can be done through the use of various equipment such as UV - vis spectroscopy, scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffractometry (XRD), Transmission electron microscope (TEM) and so on. Antibacterial activity to test Ag-NPs was done by the well diffusion method against a Gram positive (S aureus) and a Gram negative bacteria (E coli).
ABBREVIATIONS
PVA: Polyvinyl Alcohol; Ag-NP: Silver Nanoparticles.
INTRODUCTION
Nanoparticles are particles/materials that have dimensions between 1 and 100 nm in size. These nanoparticles have distinct characteristics from the bulk material's particles. For example, nanoparticles have a larger surface area as their size decreases. Surface area is important for catalytic activity and other features, such as antibacterial activity in Ag-NPs [1,2].
Chemical methods of Ag-NP synthesis include methods like chemical reduction by various organic and inorganic reducing agents such as sodium citrate and sodium borohydride. While this method of Ag-NP synthesis is quite common, green synthesis offers a much safer, cost-effective and eco-friendly alternative to chemical reduction [3,4]. Green synthesized Ag-NPs have applications in various fields such as medicine, food preservation, and water filtration. Moreover, according to recent studies, green synthesized Ag-NPs have strong anti-microbial, anticancer and antioxidant activity. One of the most serious threats to healthcare worldwide is the presence of multi-drug resistant pathogens, especially ones which cause life threatening diseases. To best these pathogens, new techniques of treatment of these infections are needed. Green synthesized Ag-NPs have been found to be effective against these multidrug resistant microorganisms.
Ag-NPs are frequently used for a variety of reasons, including their antibacterial properties and low toxicity [5]. There are two proposed approaches for measuring Ag-NP antibacterial activity. One advantage of Ag-NPs is their huge surface area, which allows them to quickly bind to and penetrate the bacteria's cell membrane. The second explanation proposes that Ag-NPs interfere with the respiratory chain of bacterial mitochondria, resulting in cell death [6]. Although there are other methods for producing Ag-NPs, plant-based synthesis is preferred because it is environmentally benign, cost-effective, and eliminates the use of harmful ingredients such as hydrazine and sodium borohydride used in chemical synthesis [5]. The biological resources used (plants such as neem and tulsi, fungi such as Aspergillus spp, Trichoderma, algae such as Chlorella, Spirulina, etc.) serve as reducing, stabilizing, and capping agents [5,7]. Ag-NPs are classified based on their structure and morphology. These include cubic, spherical, rod, triangular, disk, bar, and wire shapes. Various analytical methods are utilized to characterize size, shape, distribution, and stability.
These methods include: (1) UV-vis Spectroscopy, which describes nanoparticle production at the first stage, (2) Fourier transform infrared spectroscopy (FTIR) assists in characterizing the function of chemicals involved in the reduction and capping of AgNps, as well as the chemical residue on the surface of the Ag-NPs. (3) X-ray diffraction (XRD) is used to identify the material and determine the crystallinity. (4) Scanning electron microscopy (SEM) determines the surface shape, size, and dispersion of nanoparticles [8].
In this review, we shall attempt to provide an insight into green synthesis of Ag-NPs, its different methods, how addition of a synthetic polymer such as PVA affects the efficacy of the AgNPs and its effect on the antibacterial activity.
EXTRACTION OF PHYTOCHEMICALS FROM PLANT PARTS
Reducing agents have been widely used in biological systems. The primary requirements for green AgNP production are silver metal ion solution and a reducing biological agent. In most cases, reducing agents and other cell constituents act as stabilizers and caps, removing the need for external agents [2].
Biologically-mediated nanoparticle production provides a cost-effective and ecologically friendly alternative to chemical techniques. This approach produces nanoparticles such as silver, gold, and graphene by combining bacteria, fungi, plant extracts, and tiny biomolecules, decreasing toxicity and environmental harm. The biological synthesis of nanoparticles is dependent on three factors: (a) the solvent, (b) the reducing agent, and (c) the nontoxic substance [9].
Plants ranging from algae to angiosperms have been employed for green production of Ag-NPs, with angiosperms being the most popular. AgNP production has used plant parts like leaves, bark, roots, and stems from medicinally significant species such as Boerhaavia diffusa, Aloe vera, Catharanthus roseus, and Ocimum tenuiflorum, which operate as both reducing and stabilizing agents. In addition, exotic weeds (Parthenium hysterophorus) and other plants that produce alkaloids or essential oils have been employed. Water is usually the chosen solvent, but organic solvents (methanol, ethanol, and ethyl acetate) are also used.
Compared to plant-based techniques, microbial synthesis of AgNPs employing fungi and bacteria is more complex due to culture management and standardization issues. Microbial production can occur both extracellularly and intracellularly, but the extracellular pathway is more commonly documented. Common fungal taxa, such as Fusarium, are frequently employed for AgNP production. While some researchers use stabilizing agents such as L-cystine and piperitone, most bio-polymers in AgNP synthesis also function as reducing agents, allowing for more control over nanoparticle size and form.
There are various extraction techniques used to obtain phytochemicals from different plant types and plant parts. Each Technique varies with time, pH, temperature and the type of plant used. The various extraction methods commonly used are:
METHOD OF EXTRACTION OF PHYTOCHEMICALS
Maceration Method
Maceration is one of the easiest methods yet produces inaccurate results as it is performed manually. In this method, the plant sample is dried and powdered. It is then put in a closed vessel and a good amount of solvent is added. It is then soaked for 72 hours for better results. The phytoextract is then separated for future purposes [10].
The maceration process is divided into two stages: osmosis and diffusion. Initially, osmosis occurs swiftly, often in a matter of seconds, when the solvent travels across the cell membrane in accordance with van't Hoff's law, which causes osmotic pressure. The pressure is determined using thermodynamics and depends on solute characteristics, such as the osmotic coefficient (φs) and reflection coefficient (σs).
In the later phases, osmosis occurs concurrently with diffusion, guided by Fick's laws and Maxwell-Stefan diffusion principles. Effectiveness of tannin extraction is influenced by factors such as material size, solvent type, temperature, and stirring. Smaller material sizes complicate filtration, whereas larger particles are more suited to technologies such as microwave-assisted extraction.
The efficiency of tannin extraction is determined by factors such as solvent choice, temperature, duration, and pressure. Different solvents perform better at distinct temperatures for different plant kinds. For example, acetone frequently removes more tannins than ethanol. Optimal conditions differ based on the tannins and plant species, necessitating specialized procedures for efficient extraction.
The tannins from grape pomace were extracted at 10°C for 120 minutes. Chemicals underwent cooling, cleaning, and filtering. To precipitate tannins, use dilute HCl to continually adjust the liquid phase's pH to 1.5. Centrifugation at 8000 rpm for 15 minutes yielded precipitated tannins. The extraction yield is higher than when grape pomace is treated with a 5% (w/w) solution of sodium hydroxide [11]. Maceration, similar to infusion, involves soaking things in cold or boiling water for a brief time [12].
Decoction Method
Method of decoction is similar to that of maceration. The only difference between these two methods is that heat is involved in the decoction method. The plant sample is boiled at 90-100°C with 1:10 w/v deionised water for 15-20 minutes. This is then cooled down and filtered for further use [13].
Two extraction methods were chosen due to their ease of execution, quick processing time, cost-effectiveness, and ability to consistently deliver the desired results.
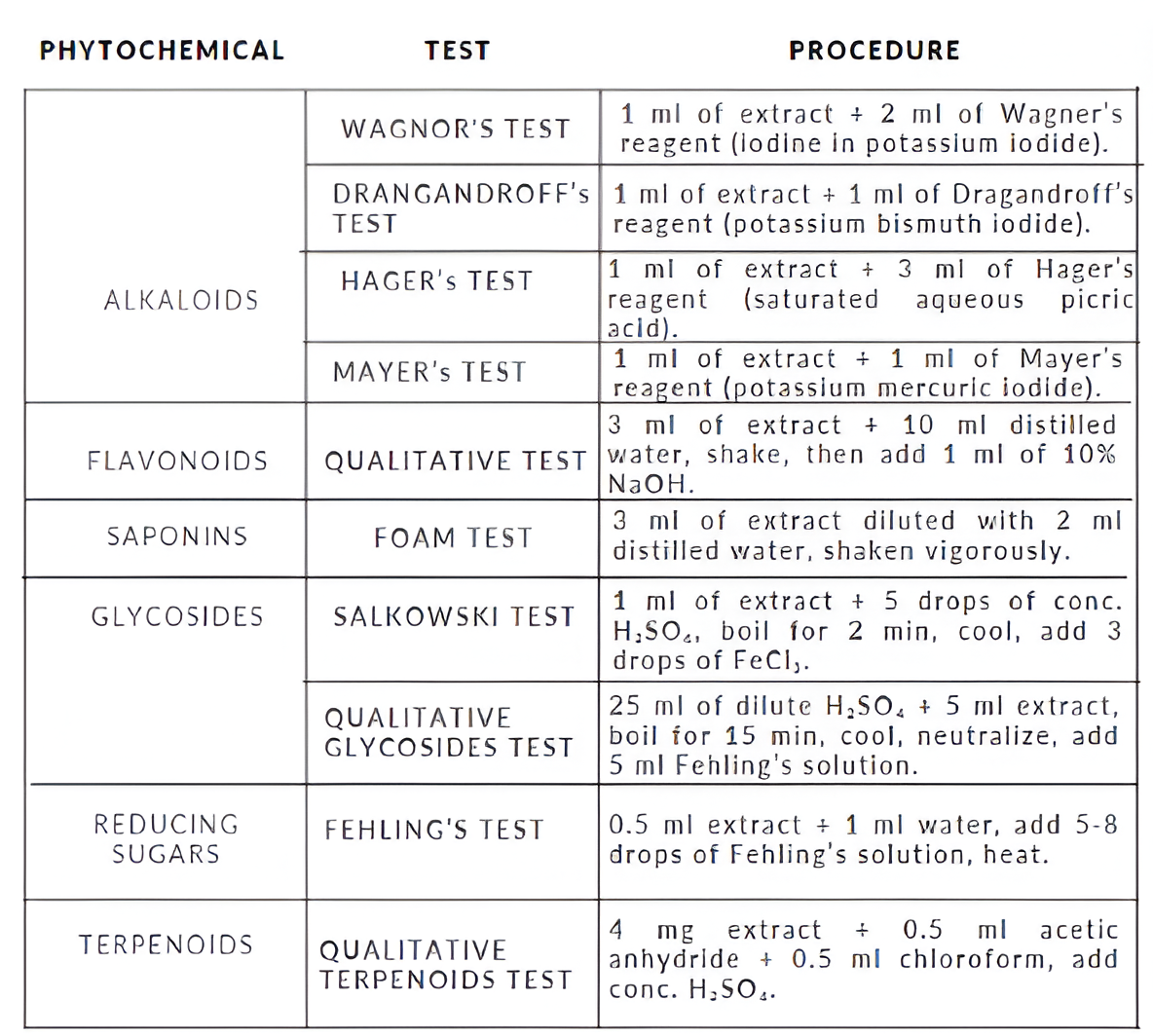
PHYTOCHEMICAL TESTING
ROLE OF PHYTOCHEMICALS IN SYNTHESIS OF Ag-NPs
Environmentally friendly production of Ag-NPs by employing readily accessible reducing and capping agents derived from plants, which are therefore renewable resources. The manufacture of nanoparticles via traditional chemical processes has drawbacks, such as high costs and detrimental effects on the environment.
Green technologies for the manufacturing of nanoparticles have emerged as environmentally benign options to address these problems. When synthesizing Ag-NPs, plant extracts are effective reducing and stabilizing agents.
It is possible to generate nanoparticles of different sizes without the use of hazardous chemicals or external stabilizing agents by replacing plant extracts.
- : Flavonoids are the constituents of plant pigments. They represent a class of secondary metabolites due to their diverse chemical and structural properties and biosynthesis in plants. Flavonoids are thought to be the main bio reducing ingredients of plant extracts, and their reducing potential is linked with their capability of donating hydrogen atoms or electrons. The process of converting silver ions (Ag⁺) into metallic silver (Ag⁰) is carried out by flavonoids. During the green synthesis process, flavonoids function as effective reducing agents.
- Alkaloids help convert Ag⁺ to Ag⁰ by contributing to the reduction process.
- Terpenoids (isoprenoids) belong to a category of naturally synthesized (low molecular weight substances) terpenes, responsible for various plant species’ aroma, taste, and color. These substances act as capping agents, preserving stability and inhibiting the agglomeration of nanoparticles. The resultant nanoparticles' size and form are mostly regulated by terpenoids.
- Saponins take part in the phases of capping and reduction. They improve the produced Ag-NPs' general stability.
- phenolic acid is an important phytochemical belonging to the polyphenol family. It has a nucleophilic aromatic ring which possesses metal-chelating ability and antioxidant activity. Phenolic chemicals ensure the biocompatibility and possible uses of the nanoparticles by stabilizing them.
- Like other phytochemicals, tannins have lowering qualities. They aid in the conversion of Ag3O to Ag3O. Furthermore, tannins have the ability to function as capping agents, which enhances the stability of the nanoparticles.
SYNTHESIS OF Ag-NPs
The essential step in synthesizing AgNPs is Ag+ ions, which can be obtained from a variety of water-soluble silver salts. Most researchers use an aqueous AgNO3 solution with an Ag+ ion concentration of 0.1 to 10 mm (usually 1 mm) [2].
Under the sonication conditions at room temperature AgNO3 solution is added to the extract. A significant color change of the extract from light brown or green to dark brown was observed showing a bioreduction process for the formation of AgNPs. The confirmation of AgNPs was carried out by measuring their absorbance by UV-Visible Spectrophotometer [13].
Factors affecting the production of AgNps are:
Concentration of extract, temperature, metal salt, pH, and contact time influence the size and properties of the AgNPs.
Stability
Nanoparticles were kept in a refrigerator at 4°C for up to 28 days, Or the nanoparticles were heated in a water bath at 80°C for 2 hours. After heating, the samples were cooled to room temperature [15].
MECHANISM OF ANTIBACTERIAL ACTIVITY OF Ag Nps
One of the main ways to understand the antibacterial activity of a system is to understand its particular modus operandi.
There are 2 steps that will affect its efficiency:
- Behavior of Ag-NPs in the vicinity of the environment of interest where physical and chemical changes can occur. This includes redox reactions, interaction with other nanoparticles or surfaces and so on all have an impact on the antibacterial activity of Ag-NPs [16].
- Interaction of Ag-NPs with the cell wall which eventually results in cellular death. This depends on the affected organism but also other parameters such as size, shape, dispersion, evaluation procedures such as bacterial stain used and composition of media. Once AgNPs come into contact with bacteria they tend to form aggregates which leads to the decrease in the cell wall integrity and these perforations lead to cell death. Another method of cytotoxic activity of AgNps is the generation of a reactive oxygen species(ROS). In these conditions the cells endure a very high level of oxidative stress which leads to cellular inactivation [6,16,17].
COATING OF Ag-NPs WITH POLYMERS
To coat Ag-NPs (AgNPs) with polymers such as starch, PVA, or propyl amine-substituted PVA, dissolve the polymer in water, mix it with AgNPs or silver salts, then cast the mixture into films. Glutaraldehyde and other cross-linking compounds are utilized to improve mechanical characteristics. The coating improves nanoparticle stability and dispersion, reduces aggregation, and boosts biocompatibility for use in biomedical areas and antimicrobial films [18].
POLY-VINYL ALCOHOL (PVA) LAYER
PVA was dissolved in water at a concentration of 4% (w/w) and stirred for one hour at 80 degrees Celsius. Once the PVA solution was entirely dissolved and homogenous, a colloidal suspension of preformed Ag-NPs (AgNP) was gradually added to achieve various compositions comprising Ag nanoparticles ranging from 1% to 5% by weight.
The resulting mixture was thoroughly mixed to ensure that the Ag nanoparticles were evenly distributed throughout the PVA matrix. Thin films of these PVA/AgNP solutions were then formed onto glass plates and allowed to cure before further investigation.
The findings of this study focus on compositions including 1% w/w Ag nanoparticles within the PVA matrix [19-21].
ANTIBACTERIAL ACTIVITY
Upon successful synthesis of AgNps and their conjugation with the phytochemicals from plant extract and PVA we must check the antibacterial properties of the samples namely AgNP alone, AgNP+plant extract, AgNP+plant extract+PVA and finally control (distilled water). These samples are checked using a Gram positive bacteria (Staphylococcus aureus) and a Gram negative bacteria (Escherichia coli), through two main methods: well diffusion method and disc diffusion method where disc diffusion method is used to determine how susceptible bacteria are to an antibiotic and well diffusion method is used to evaluate the antimicrobial activity of plant extracts.
Well diffusion method
The antibacterial activity of AgNPs were observed against Gram positive bacteria (Staphylococcus aureus) and Gram negative bacteria (Escherichia coli) through well diffusion method. This is done by comparing the sizes (in mm) of the zones of inhibition of the different samples taken. The extent of the halo or ring formed around the sample/solution gives an indication to the level of the antibacterial activity of the sample [22].
Disc method
Disc diffusion method is another technique through which antibacterial activity of Ag-NPs can be measured wherein the nanosilver shapes were cut into a disc shape and sterilized by autoclaving (15 min at 121°C), and was placed on E coli cultured agar plate and S aureus cultured agar plate. The agar plates were then incubated for 24 h at 37°C. After incubation the presence and size of the inhibition zone (in mm) was observed and measured [22].
CHARACTERISATION
The characterisation of the nanoparticles can be done through the use of various equipment such as UV - vis spectroscopy, scanning electron microscope(SEM), Fourier transform infrared spectroscopy(FTIR), X-ray diffractometry(XRD), Transmission electron microscope(TEM). These techniques are vital tools in nanotechnology for thoroughly 0-understanding nanoparticles, and we'll go into more detail on each of them in the following sections [8,23,24].
Uv vis spectro
UV-Vis spectroscopy is an essential and commonly employed technique for the initial assessment of synthesized nanoparticles, such as Ag-NPs (AgNPs) or any other. This technique is especially useful because it can track the synthesis process and evaluate the stability of nanoparticles. Due to their distinctive properties, AgNPs can strongly interact with certain wavelengths of light, which makes UV-Vis spectroscopy particularly suitable for analyzing these particles, and by analyzing the graph we can make sure that nanoparticles are synthesized [25].
Although the color change is a good visual sign of Ag-NP (Ag-NP) formation, UV-Vis spectroscopy provides more precise confirmation. When using this method, Ag-NPs created through both green and chemical synthesis methods show distinct surface plasmon resonance (SPR) peaks. We can vary the wavelength and get the plots. The peaks, particularly the strong absorption around 400 nm, indicate successful nanoparticle synthesis by highlighting how free electrons interact with light.
When we compare for chemically synthesized Ag-NPs and the green synthesized nanoparticles in chemically synthesized, the SPR peaks are read and in this intense and slightly shift to lower wavelengths occur which suggests that these nanoparticles are smaller and more uniform. In the green synthesis process the changes in the peak values suggest that starch plays a key role in determining the size, reduction process, and stabilization of the nanoparticles. Furthermore, the broad absorption range seen in PVA-encapsulated Ag-NPs, with a noticeable curve around 400 nm, confirms the presence of the nanoparticles [18,19].
Sem (scanning electron microscope)
Using Scanning Electron Microscopy (SEM) we can closely examine the size, structure and distribution details of Ag-NPs (Ag-NPs) and also in various polymers.in the scanning electron microscope magnification ranges from 20x to 30,000x, and the area from range of 1cm to 5μm can be imaged by the SEM. The samples are supposed to centrifuged and dried and then these samples are used accordingly in the analysis, for the polymer encapsulated Ag-NP the samples are dried and analyzed these images show that pure carboxymethyl cellulose (CMC) undergoes a transformation in the form of square-like structures with Ag-NPs nestled in the center. When Ag-NPs are encapsulated by polymers like PVA (PVA) and polypyrrole, the polymers coat the particles, which enhances their stability and functionality.
For example, SEM studies reveal that Ag-NPs produced from the green synthesis methods are smaller around 19 nm when compared to chemical synthesis they are about 40 nm. This difference is due to the starch which was used in the green method, starch encourages the formation of smaller nanoparticles. In films made from PVA and PA-PVA, the Ag-NPs are well distributed at lower concentrations, but some clustering is observed at higher concentrations [18].
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR is a versatile and affordable tool for studying the surface of nanoparticles, helping to identify both their chemical composition and reactive surface sites. It allows us to understand how the nanoparticles behave chemically and where reactions occur on their surface. FTIR is especially useful in green synthesis, revealing how biological molecules reduce Ag⁺ ions and stabilize the Ag-NPs. This makes it a key technique for characterizing and controlling nanoparticle properties [26].
This is an important and affordable tool for studying the surface of nanoparticles; this characterisation technique helps to identify both their chemical composition and reactive surface sites. It allows us to understand how the nanoparticles behave chemically and where reactions occur on their surface. FTIR is especially useful in green synthesis, revealing how biological molecules reduce Ag⁺ ions and stabilize the Ag-NPs. This makes it a key technique for characterizing and controlling nanoparticle properties [26].
The FTIR spectrum of starch-capped Ag-NPs reveals key changes in the natural bands of starch, indicating a strong interaction between starch and the nanoparticles. When it comes to polymer encapsulation to the nanoparticle the stretch is analysed. The OH stretching, typically seen at (3143 cm⁻¹) and the carbonyl stretching at (1634 cm⁻¹) this show noticeable shifts which suggests that starch is actively involved in capping the nanoparticles. Similarly, the shifting of the COH bend at 981 cm⁻¹ and the C–O–C symmetrical stretching at 1348 cm⁻¹ further support the link between AgNPs and starch. These alterations show how the starch becomes more stable the nanoparticles and keeps them from clumping [18].
This is a tool for analyzing nanoparticles, providing detailed information on particle size, distribution, shape, and morphology. By using an electron beam, TEM captures high-resolution images that are useful in analyzing nanoparticles. They are prepared to withstand the electron beam by which the clear images are taken. These images provide valuable information about the size and arrangement of the nanoparticles, helping us understand their properties [8,24].
Transmission Electron Microscopy (TEM)
TEM has a better resolution and the screened image and it provides us the data, but TEM has some of the drawbacks such as small sample size requirements, high vacuum space, sample preparation and it takes time for this process.the samples are analyzed by the magnification between objective lens, the sample and the image plane.another most commonly used term (SAED) Selected Area Electron Diffraction this is used to analyze the crystalline structure of the Ag-NPs which provides the data and helps in understanding the composition of the nanoparticle [26].
In the TEM image of pure Ag-NPs can be analyzed, the smallest particles are about 20 nm, with some larger ones between 20 and 50 nm due to clustering when screened in this paper. When Ag-NPs are encapsulated in a polymer like PVA or any other polymer, the TEM image reveals a core-shell structure where the nanoparticles are embedded in the polymer matrix. This appears different from the only polymer, which usually appears as larger, spherical or globular particles averaging around 0.8 microns. The polymer encapsulation creates a more defined and organized structure compared to the un encapsulated nanoparticles [25].
X-Ray Diffraction (XRD)
X-Ray Diffraction (XRD) is a key tool for studying the size and structure of Ag-NPs. Through the analysis of the diffraction patterns that result when X-rays interact with the sample, XRD gives us important details about the nanoparticles' crystalline structure. This helps us understand how the nanoparticles are arranged and their size, providing valuable information for various applications [24].
The data shows that the polymer effectively encapsulates the Ag-NPs. The XRD pattern reveals peaks around 20° that come from the crystalline PVA, and clear peaks at 38°, 44°, 64°, and 78° which are characteristic of the Ag-NPs’ cubic structure. This indicates that the Ag-NPs are well-integrated into the polymer matrix [25].
Dynamic Light Scattering (DLS)
Dynamic Light Scattering is a technique used to measure the surface charge, size, and distribution of nanoparticles. It works by analyzing how light scattered by nanoparticles moves in response to their Brownian motion in a colloidal solution. This data helps estimate the hydrodynamic diameter of the particles. While DLS can measure particles ranging from 1 to 500 nm, it struggles with large agglomerated particles and is best for monitoring early-stage aggregation. Because it’s sensitive to aggregates, DLS works well when only a few particles are present to minimize scattering effects [26].
Scope and Applications
Ag-NPs have a wide range of applications as antibacterial agents in the health industry, food packaging, textile industry and in the environmental industry due to their effectiveness in attacking pathogens. In the textile industry, silver nanocomposite fibres were prepared with Ag-NPs incorporated into it. These fibers exhibited high antimicrobial activity. Furthermore, electrochemical properties of Ag-NPs are used in nano scale sensors for faster response time [53]. Ag-NPs are also effective drug delivery systems and drug carrying systems [27-54].
CONCLUSION
Green synthesis of Ag-NPs provides a clean, eco-friendly and more cost effective way to produce Ag-NPs when compared with chemical methods of producing them. Synthesis, characterisation, and application of Ag-NPs has immense potential in various areas such as antibacterial agents. Using plant extracts (green synthesis) grants a sustainable and eco-friendly method for the production of Ag-NPs. Additionally, stabilizing agents like PVA provide support in the way of preventing aggregation, and extending the shelf life of nanoparticles. Techniques and devices such as UV-vis spectroscopy, SEM, FTIR and XRD are used to confirm the successful synthesis of Ag-NPs, their dimensions and stability allowing for further study. Green synthesized Ag-NPs show strong antibacterial activity due to the presence of compounds such as phenols and flavonoids. Confirmation of the antibacterial activity is done through well diffusion method against Gram positive and Gram negative bacteria.
REFERENCES
- Song JY, Kim BS. (2008). Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess and Biosystems Engineering. Springer Science and Business Media LLC; 32(1):79-84.
- Srikar SK, Giri DD, Pal DB, Mishra PK, Upadhyay SN. (2016). Green Synthesis of Silver Nanoparticles: A Review. Green and Sustainable Chemistry. Scientific Research Publishing, Inc. 06(01):34-56.
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. (2014). Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 9(6):385-406.
- Huq Md A, Ashrafudoulla Md, Rahman MM, Balusamy SR, Akter S. (2022). Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers. MDPI AG. 14(4):742.
- Farooqui MA, Chauhan PS, Krishnamoorthy P, Shaik J. (2010). Extraction of Silver Nanoparticles from the Leaf Extracts of Clerodendrum inerme. Digest Journal of Nanomaterials and Biostructures. 5(1):43-49.
- Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. (2016). Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 12(3):789-799.
- Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. (2015). Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C Mater Biol Appl. 53:298-309.
- Kaabipour S, Hemmati S. (2021). A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J Nanotechnol. 12:102-136.
- Zhang XF, Liu ZG, Shen W, Gurunathan S. (2016). Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int J Mol Sci. 17(9):1534.
- Cuong DX, Tuyen DTT, Ha HT, Thuy LTM, Van Thanh N, Dong DH, Hoan NX, & Chinh DX. (2018). Tannins. http://books.google.ie/books?id=Ggwi0AEACAAJ&dq=Chapter+Tannins:+Extraction+from+Plants+Dang+Xuan+Cuong,+Nguyen+Xuan+Hoan,+Dinh+Huu+Dong,+Le+Thi+Minh+Thuy,+Nguyen+Van+Thanh,+Hoang+Thai+Ha,+Dang+Thi+Thanh+Tuyen+and+Dang+Xuan+Chinh&hl=&cd=1&source=gbs_api
- Noureddine B, Mohamed A,Mohamed KS, Ramzi K. (2017). Properties of tannin-glyoxal resins prepared from lyophilized and condensed tannin. J Textile Eng Fashion Technol. 3(4):705-711.
- Bimakr M. (2010). Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food and Bioproducts Processing. 89(1):67-72.
- Rajput D, Paul S, Gupta A. (2020). Green Synthesis of Silver Nanoparticles Using Waste Tea Leaves. Advanced Nano Research. 3(1):1-14.
- Inamdar P, Vazir J, Desai S, Patel D, Meshram D. (2014). Phytochemical screening and in vitro antifungal activity of Camellia sinensis. International Journal of Pharmacy and Pharmaceutical Sciences. 6(5):148-150.
- Liang X, Cheng W, Liang Z, Zhan Y, McClements DJ, Hu K. (2022). Co-Encapsulation of Tannic Acid and Resveratrol in Zein/Pectin Nanoparticles: Stability, Antioxidant Activity, and Bioaccessibility. Foods. 11(21):3478.
- Ouay BL, Stellacci F. (2015). Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today. 10(3):339-354.
- Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB. (2010). Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 85(4):1115-1122.
- Iqbal M, Zafar H, Mahmood A, Niazi MBK, Aslam MW. (2020). Starch-Capped Silver Nanoparticles Impregnated into Propylamine-Substituted PVA Films with Improved Antibacterial and Mechanical Properties for Wound-Bandage Applications. Polymers (Basel). 12(9):2112.
- Chandran S, Ravichandran V, Chandran S, Chemmanda J, Chandarshekar B. (2016). Biosynthesis of PVA encapsulated silver nanoparticles. Journal of Applied Research and Technology. 14:319-324.
- Ernest Ravindran R, Subha V, Ilangovan R. (2020). Silver nanoparticles blended PEG/PVA nanocomposites synthesis and characterization for food packaging. Arabian Journal of Chemistry. 13(7):6056-6060.
- Chitte HK, Bhat NV, Karmakar NS, Kothari DC, Shinde GN. (2012). Synthesis and characterization of polymeric composites embedded with silver nanoparticles. World Journal of Nano Science and Engineering. 2(01):19.
- Sadeghi B, Jamali M, Kia SH, Nia AA, Ghafari S. Synthesis and characterization of silver nanoparticles for antibacterial activity. International Journal of Nanodimension. 1(2):119-124.
- Dawadi S, Katuwal S, Gupta A, Lamichhane U, Thapa R, Jaisi S, et al. (2021). Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. Journal of Nanomaterials. 2021:1-23.
- Anith Jose R, Devina Merin D, Arulananth TS, Shaik N. (2022). Characterization Analysis of Silver Nanoparticles Synthesized from Chaetoceros calcitrans. Journal of Nanomaterials. 2022:1-15.
- Chitte HK, Bhat NV, Karmakar NS, Kothari DC, Shinde GN. (2012). Synthesis and Characterization of Polymeric Composites Embeded with Silver Nanoparticles. World Journal of Nano Science and Engineering. 02(01):19-24.
- Akhter MS, Rahman MA, Ripon RK, Mubarak M, Akter M, Mahbub S, et al. (2024). A systematic review on green synthesis of silver nanoparticles using plants extract and their bio-medical applications. Heliyon. 10(11):e29766.
- Burdușel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. (2018). Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials (Basel). 8(9):681.
- Hossain MA, Ahmad NU, Alam M, Hossain MM, Sarkar A. (2024). Screening of different extraction methods for maximum production of total flavonoids, tannins, and antioxidants from Centella asiatica. Food Research. Rynnye Lyan Resources. 8(1):44-51.
- Das AK, Islam MdN, Faruk MdO, Ashaduzzaman Md, Dungani R. (2020). Review on tannins: Extraction processes, applications and possibilities. South African Journal of Botany. Elsevier BV. 135:58-70.
- Aguilera JR, Venegas V, Oliva JM, Sayagués MJ, de Miguel M, Sánchez-Alcázar JA, et al. (2016). Targeted multifunctional tannic acid nanoparticles. RSC Advances. Royal Society of Chemistry (RSC). 6(9):7279-7287.
- Fraga-Corral M, García-Oliveira P, Pereira AG, Lourenço-Lopes C, Jimenez-Lopez C, Prieto MA, et al. (2020). Technological Application of Tannin-Based Extracts. Molecules. MDPI AG. 25(3):614.
- Si Heung Sung. Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. Journal of Medicinal Plants Research. Academic Journals. 6(15).
- Cường ĐX, Xuan HN, Dong DH, Thủy LTM, Thành NV, Ha HT, Tuyen DTT, Chinh DX. (2020). Tannins: Extraction from Plants [Internet]. IntechOpen eBooks. Available at: http://dx.doi.org/10.5772/intechopen.86040
- Logeswari P, Silambarasan S, Abraham J. (2015). Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. Journal of Saudi Chemical Society [Internet]. Elsevier BV. 19(3):311-317.
- Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, et al. (2018). Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artificial Cells, Nanomedicine, and Biotechnology. Informa UK Limited. 46(sup3):855-872.
- Ajitha B, Ashok Kumar Reddy Y, Sreedhara Reddy P. (2015). Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Materials Science and Engineering: C. Elsevier BV. 49:373-381.
- Tongwanichniyom S, Phewrat N, Rangsarikorn N, Leasen S, Luangkamin S, Chumnanvej N. (2024). Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities. Green Processing and Synthesis. Walter de Gruyter GmbH. 13(1).
- El-Desouky N, Shoueir K, El-Mehasseb I, El-Kemary M. (2022). Synthesis of silver nanoparticles using bio valorization coffee waste extract: photocatalytic flow-rate performance, antibacterial activity, and electrochemical investigation. Biomass Conversion and Biorefinery. Springer Science and Business Media LLC. 13(17):15871-15885.
- Khan Z, Al-Thabaiti SA. (2019). Biogenic silver nanoparticles: Green synthesis, encapsulation, thermal stability and antimicrobial activities. Journal of Molecular Liquids. 289:111102.
- Wang T, Zhang F, Zhao R, Wang C, Hu K, Sun Y, et al. (2020). Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Designed Monomers and Polymers. 23(1):118-133.
- Vimala K, Yallapu MM, Varaprasad K, Reddy NN, Ravindra S, Naidu NS, et al. (2011). Fabrication of Curcumin Encapsulated Chitosan-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. Journal of Biomaterials and Nanobiotechnology. 02(01):55-64.
- Mohanaparameswari S, Balachandramohan M, Sasikumar P, Rajeevgandhi C, Vimalan M, Pugazhendhi S, et al. (2023). Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method. Green Processing and Synthesis. 12(1).
- Cataldo F. (2014). Green synthesis of silver nanoparticles by the action of black or green tea infusions on silver ions. Eur Chem Bull. 3(3):280-289.
- Mailoa MN, Mahendradatta M, Laga A, Djide N. (2013). Tannin Extract of Guava Leaves (Psidium Guajava L) Variation With Concentration Organic Solvents. International Journal of Scientific & Technology Research. 2(9):106.
- Baldosano HY, Castillo MBMG, Elloran CDH, Bacani FT. (2015). Effect of Particle Size, Solvent and Extraction Time on Tannin Extract from Spondias purpurea Bark Through Soxhlet Extraction. Presented at the DLSU Research Congress 2015, De La Salle University, Manila, Philippines.
- Liang X, Cheng W, Liang Z, Zhan Y, McClements D, Hu K. (2022). Co-Encapsulation of Tannic Acid and Resveratrol in Zein/Pectin Nanoparticles: Stability, Antioxidant Activity, and Bioaccessibility. Foods. 11(21):3478.
- Basak P, Das P, Biswas S, Biswas NC, Mahapatra GKD. (2018). Green Synthesis and Characterization of Gelatin-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. IOP Conference Series: Materials Science and Engineering. 410(1):012019.
- Okafor F, Janen A, Kukhtareva T, Edwards V, Curley M. (2013). Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int J Environ Res Public Health. 10(10):5221-5238.
- Ullah AKMA, Kabir MF, Akter M, Tamanna AN, Hossain A, Tareq ARM, et al. (2018). Green synthesis of bio-molecule encapsulated magnetic silver nanoparticles and their antibacterial activity. RSC Adv. 8(65):37176-37183.
- Kardel M, Taube F, Schulz H, Schütze W, Gierus M. (2013). Different approaches to evaluate tannin content and structure of selected plant extracts – review and new aspects. Journal of Applied Botany and Food Quality. 86(1):154-166.
- Al-Ogaidi I, Salman MI, Mohammad FI, Aguilar Z, Al-Ogaidi M, Hadi YA, et al. Antibacterial and Cytotoxicity of Silver Nanoparticles Synthesized in Green and Black Tea. World J Exp Biosci. 5(1):39-45.
- Khan Z, Al-Thabaiti SA. (2019). Biogenic silver nanoparticles: Green synthesis, encapsulation, thermal stability and antimicrobial activities. Journal of Molecular Liquids. 289(2):111102.
- Yashin AY, Nemzer BV, Combet E, Yashin YI. Determination of the Chemical Composition of Tea by Chromatographic Methods: A Review. Journal of Food Research. 4(3):56.
- Huq MA, Ashrafudoulla M, Rahman MM, Balusamy SR, Akter S. (2022). Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers (Basel). 14(4):742.
- El-Nour KMMA, Eftaiha A, Al-Warthan A, Ammar RAA. Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry. 3(3):135-140.
 Abstract
Abstract  PDF
PDF